Be The Molecule
Gas pressure is caused by the collisions of molecules or atoms with the walls of the container they are in. Pressure changes when either the volume of the container changes or the molecules' temperature changes. In the first case, the same amount of molecules are suddenly confined in a small space, so they collide with each other and the walls more often, creating more pressure. In the second case, the increased temperature makes the individual molecules move faster, so once again they collide with the walls more often and create more pressure.
When you fly in an airplane, you can feel the change of pressure in your ears when you ascend to higher altitudes. This is because the higher you are, the lower the air pressure, and the air trapped in your inner eardrum is suddenly high pressure in comparison. Because of this, it exerts pressure on your ear, causing the painful feeling that tells you you're high in the air.
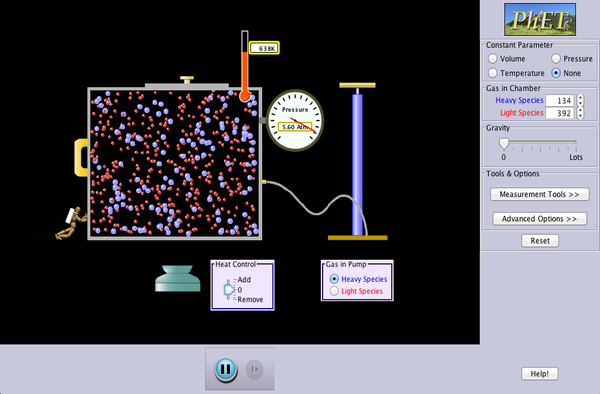 In class this week we conducted an online pHet simulation, exploring the effects of heat, volume of container, and quantity on molecules. In the simulation, there was box with a lid, into which we could pump molecules. This box was re-sizable and could be heated or cooled. With these tools, my lab partner and I explored how many different ways we could blow the lid of the container -- increase the pressure in the box enough for that to occur.
In class this week we conducted an online pHet simulation, exploring the effects of heat, volume of container, and quantity on molecules. In the simulation, there was box with a lid, into which we could pump molecules. This box was re-sizable and could be heated or cooled. With these tools, my lab partner and I explored how many different ways we could blow the lid of the container -- increase the pressure in the box enough for that to occur.We discovered three ways of doing this, two of which support my earlier statement that pressure increases when volume decreases or temperature increases. First, we pumped a single molecule into the box, then heated it up until it hit the lid so frequently that it was blown off. This shows that enough heat, on its own, can cause drastic changes in pressure. Second, we pumped in ten molecules, then made the box as small as possible. This made the molecules collide with the walls far more frequently, and showed that decreased volume can also cause major changes in pressure, Finally, we just pumped in huge amounts of molecules, which quickly caused the lid to be blown off. This last method didn't really support a separate way pressure increases, because it really just showed a larger scale version of decreased volume causing an increase in pressure,
Pressure Systems
Areas of high and low pressure are directly related to fronts. Fronts are where warm and cool air masses meet - when they meet, the warm air rises, leaving an area of low pressure below. These areas of low pressure are associated with clouds, precipitation, and storms, because the reduced weight of air above creates better conditions for these weather events to form.
Areas of high and low pressure are also related to the jet stream. The jet stream separates areas of high and low pressure -- weaving around, it ducks south areas of low pressure and north of areas of high pressure. As well, the curve of the jet stream nearly matches the curves of isobars, which are lines that mark areas with the same air pressure.
This week, we discussed why, even though heat rises, the atmosphere is cold -- a statement which at first seems counter-intuitive. This is because, as heated molecules gain altitude they lose kinetic energy and cool down. This is also seen in the convection process -- molecules are heated from below and rise then eventually lose kinetic energy, and sink.
Cloud 101
Clouds form through a complicated process. First, there is water, perhaps a lake. Then, when the air is dry and warm, some water evaporates, or becomes vapor. If this vapor is warm enough, it rises, because it's less dense. Eventually, the vapor gets high enough and cools down, condensing and becoming the mass of water droplets we see as clouds in the sky.
You cannot drown in a cloud. It's like fog -- the condensed water vapor isn't enough to suffocate you, and it's intermingled with air. If the water droplets were dense enough to drown you, they would be precipitation. So, don't worry if you're skydiving -- you won't drown on the way down.




