Introduction:
The smallest unit of a substance, which still retains the characteristics of that substance, is
called a molecule. Molecules can only be divided into atoms - which are different
elements. For example, all molecules of water are identical and have the characteristics
of water. Two atoms of hydrogen and an atom of oxygen (which made up the molecule)
on their own have none of the characteristics of water.
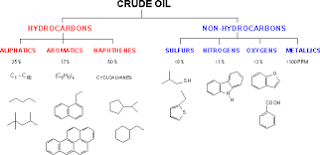
Crude oils are mixtures of many different substances, often difficult to separate, from
which various petroleum products are derived, such as: gasoline, kerosene propane, fuel
oil, lubricating oil, wax, and asphalt. These substances are mainly compounds of only
two elements: carbon (C) and hydrogen (H). They are called, therefore: hydrocarbons.
Refining crude oil involves two kinds of processes to produce the products so essential to
modern society. First, there are physical processes which simply refine the crude oil
(without altering its molecular structure) into useful products such as lubricating oil or
fuel oil. Second, there are chemical or other processes which alter the molecular structure
and produce a wide range of products, some of them known by the general term
petrochemicals.
Hydrocarbons
Hydrocarbons may be gaseous, liquid, or solid at normal temperature and pressure,
depending on the number and arrangement of the carbon atoms in their molecules. Those
with up to 4 carbon atoms are gaseous; those with 20 or more are solid; those in between
are liquid. Crude oils are liquid but may contain gaseous or solid compounds (or both) in
solution. The heavier a crude oil (i.e. the more carbon atoms its molecules contain) the
closer it is to being a solid and this may be especially noticeable as its temperature cools.
Light oils will remain liquid even at very low temperatures.
Although hydrocarbons consist of two elements only (carbon and hydrogen), they exist in
a wide variety of types and in large numbers. This arises from the ability of carbon atoms
to form long chains. The hydrocarbons may be classified according to their composition
(type and number of atoms) and the structure (arrangements of atoms in space) of the
molecule.
Hydrocarbons are usually classified in the paraffin, unsaturated, naphtene and aromatic
types.Paraffin Series
This series, also known as alkane series, is characterized by the fact that the carbon
atoms are arranged in open chains (not closed rings) and are joined by single bonds. The
hydrocarbons of the paraffin type are thus saturated (single bonds only between carbon
atoms) and have the general formula CnH2n+2.
The simplest hydrocarbon is methane, a gas consisting of one carbon atom and four
hydrogen atoms:
A carbon atom has four bonds that can unite with either one or more other carbon atoms
(a property almost unique to carbon) or with atoms of other elements. A hydrogen atom
has only one bond and can never unite with more than one other atom. The larger
hydrocarbon molecules have two or more carbon atoms joined to one another as well as
to hydrogen atoms. The carbon atoms may link together in a straight chain, a branched
chain, or a ring.
The first three members of the paraffin series methane, propane and butane respectively
have a single structural formula.
The remaining members may have two or more structural formulas for the same chemical
formula. The phenomenon, known as isomerism, has a strong impact on the
thermodynamic properties of the hydrocarbons. An example of a branched chain,
Isobutane (C4H10)
Isobutene has a boiling point of 109 oF while normal butane boils at 31.1 oF.
The members of the paraffin series are very important constituents of crude oil. Some
crude oils are largely composed of hydrocarbons of this series while others contain them
to a lesser extent. Natural gas consists mainly of the more volatile members of the
paraffin series containing from one to four carbon atoms per molecule.
The paraffin series are characterized by their chemical inertness. They will not react with
concentrated sulphuric or nitric acid at room temperature. However, when ignited on the
presence of air or oxygen, they give off large amounts of heat and under proper
conditions, this combustion is explosive. The reaction with oxygen occurs only at
elevated temperatures. The inertness of the paraffin hydrocarbons accounts for their
presence in petroleum since their existence for geological periods of time would require a
high degree of stability.
Unsaturated Hydrocarbons
The unsaturated hydrocarbons are characterized by the presence of double or triple bonds
between the carbon atoms. The multiple bonds allow the addition of hydrogen atoms,
under appropriate conditions, which explains the name unsaturated. The olefin series of
hydrocarbons is characterized by the presence of a double bond in the molecule and has
the general formula CnH2n.
The first three members (n=1…4) of this series, ethene, propene and butene are now
commonly referred to using their traditional names ethylene, propylene and butylene.
Isomerism occurs also with the olefins, not only due to the branching of the carbon
chains, but also to the position of the double bond in the molecule.
Another series of unsaturated hydrocarbons is known as diolefins. They are characterized
by the fact that there are two double bonds in the molecule. The general formula for the
series is CnH2n-2.
A third series of unsaturated hydrocarbons of considerable importance is the acetylene
series. The compounds have a triple bond and general formula CnH2n -2 . Hence they are
isomers with the diolefins. The first three members of this series (n=2…4) are ethine
(commonly called acetylene), propine and butine.
The smallest unit of a substance, which still retains the characteristics of that substance, is
called a molecule. Molecules can only be divided into atoms - which are different
elements. For example, all molecules of water are identical and have the characteristics
of water. Two atoms of hydrogen and an atom of oxygen (which made up the molecule)
on their own have none of the characteristics of water.
Crude oils are mixtures of many different substances, often difficult to separate, from
which various petroleum products are derived, such as: gasoline, kerosene propane, fuel
oil, lubricating oil, wax, and asphalt. These substances are mainly compounds of only
two elements: carbon (C) and hydrogen (H). They are called, therefore: hydrocarbons.
Refining crude oil involves two kinds of processes to produce the products so essential to
modern society. First, there are physical processes which simply refine the crude oil
(without altering its molecular structure) into useful products such as lubricating oil or
fuel oil. Second, there are chemical or other processes which alter the molecular structure
and produce a wide range of products, some of them known by the general term
petrochemicals.
Hydrocarbons
Hydrocarbons may be gaseous, liquid, or solid at normal temperature and pressure,
depending on the number and arrangement of the carbon atoms in their molecules. Those
with up to 4 carbon atoms are gaseous; those with 20 or more are solid; those in between
are liquid. Crude oils are liquid but may contain gaseous or solid compounds (or both) in
solution. The heavier a crude oil (i.e. the more carbon atoms its molecules contain) the
closer it is to being a solid and this may be especially noticeable as its temperature cools.
Light oils will remain liquid even at very low temperatures.
Although hydrocarbons consist of two elements only (carbon and hydrogen), they exist in
a wide variety of types and in large numbers. This arises from the ability of carbon atoms
to form long chains. The hydrocarbons may be classified according to their composition
(type and number of atoms) and the structure (arrangements of atoms in space) of the
molecule.
Hydrocarbons are usually classified in the paraffin, unsaturated, naphtene and aromatic
types.Paraffin Series
This series, also known as alkane series, is characterized by the fact that the carbon
atoms are arranged in open chains (not closed rings) and are joined by single bonds. The
hydrocarbons of the paraffin type are thus saturated (single bonds only between carbon
atoms) and have the general formula CnH2n+2.
The simplest hydrocarbon is methane, a gas consisting of one carbon atom and four
hydrogen atoms:
A carbon atom has four bonds that can unite with either one or more other carbon atoms
(a property almost unique to carbon) or with atoms of other elements. A hydrogen atom
has only one bond and can never unite with more than one other atom. The larger
hydrocarbon molecules have two or more carbon atoms joined to one another as well as
to hydrogen atoms. The carbon atoms may link together in a straight chain, a branched
chain, or a ring.
The first three members of the paraffin series methane, propane and butane respectively
have a single structural formula.
The remaining members may have two or more structural formulas for the same chemical
formula. The phenomenon, known as isomerism, has a strong impact on the
thermodynamic properties of the hydrocarbons. An example of a branched chain,
Isobutane (C4H10)
Isobutene has a boiling point of 109 oF while normal butane boils at 31.1 oF.
The members of the paraffin series are very important constituents of crude oil. Some
crude oils are largely composed of hydrocarbons of this series while others contain them
to a lesser extent. Natural gas consists mainly of the more volatile members of the
paraffin series containing from one to four carbon atoms per molecule.
The paraffin series are characterized by their chemical inertness. They will not react with
concentrated sulphuric or nitric acid at room temperature. However, when ignited on the
presence of air or oxygen, they give off large amounts of heat and under proper
conditions, this combustion is explosive. The reaction with oxygen occurs only at
elevated temperatures. The inertness of the paraffin hydrocarbons accounts for their
presence in petroleum since their existence for geological periods of time would require a
high degree of stability.
Unsaturated Hydrocarbons
The unsaturated hydrocarbons are characterized by the presence of double or triple bonds
between the carbon atoms. The multiple bonds allow the addition of hydrogen atoms,
under appropriate conditions, which explains the name unsaturated. The olefin series of
hydrocarbons is characterized by the presence of a double bond in the molecule and has
the general formula CnH2n.
The first three members (n=1…4) of this series, ethene, propene and butene are now
commonly referred to using their traditional names ethylene, propylene and butylene.
Isomerism occurs also with the olefins, not only due to the branching of the carbon
chains, but also to the position of the double bond in the molecule.
Another series of unsaturated hydrocarbons is known as diolefins. They are characterized
by the fact that there are two double bonds in the molecule. The general formula for the
series is CnH2n-2.
A third series of unsaturated hydrocarbons of considerable importance is the acetylene
series. The compounds have a triple bond and general formula CnH2n -2 . Hence they are
isomers with the diolefins. The first three members of this series (n=2…4) are ethine
(commonly called acetylene), propine and butine.
The unsaturated hydrocarbons are very reactive, in contrast with the members of the
paraffin series. They react rapidly with chlorine to form oily liquids ; hence the name
olefins (oil forming). Under the proper conditions they react rapidly with hydrogen,
which saturates the double bonds and forms the corresponding paraffin. Because of their
high reactivity, these unsaturated hydrocarbons are not found in crude oil to any great
extent. However, they are formed in large amounts in petroleum cracking processes and
have considerable industrial importance.
Naphthene Hydrocarbons
The naphthene hydrocarbons are also called cycloparaffins and, as ther name implies,
they are saturated hydrocarbons in which the carbon chains form closed rings. The
general formula for this series is CnH2n (n greater than 2) and consequently they are
isometric with the olefins. They are named by placing the prefix cyclo before the names
of the corresponding paraffin hydrocarbon. The first members of this series (n=3…6) are
cyclopropane, cyclobutane and cyclohexane, and so on. These compounds, being
saturated, are relatively stable and are important constituents of crude oil. In general, the
chemical properties of these hydrocarbons are very similar to those of the paraffins.
Aromatic Hydrocarbons
These hydrocarbons are also cyclic and may be considered to be derivatives of benzene
and have general formula CnH2n-6 (n greater than 5). Benzene has the formula C6H6, and
the structure consists of a six-fold ring, with alternate single and double bonds. This
structure is so common in organic compounds that chemists use a hexagon with a circle
in the middle as a special symbol to represent the benzene molecule. Some of the simpler
members of this series consist of benzene with one or more alkyl groups as side chains.
An example, methylbenzene, also known as toluene, is of sufficient importance to warrant
a common name.
The fact that the benzene ring contains three double bonds suggests that the members of
this series should be very reactive. However, this is not so and, although they are not as
stable as the paraffins, they do not show the high reactivity that is so characteristic of the olefins. Compounds of this series do occur in crude oil. Petroleum is one of the important
sources of these important hydrocarbons.
The aromatic hydrocarbons are either liquids or solids under standard conditions of
temperature and pressure. Benzene is a colorless liquid with as boiling point of 176oF.
Many of the members of this series are characterized by fragrant odors; hencr the name
aromatic given to this series.
paraffin series. They react rapidly with chlorine to form oily liquids ; hence the name
olefins (oil forming). Under the proper conditions they react rapidly with hydrogen,
which saturates the double bonds and forms the corresponding paraffin. Because of their
high reactivity, these unsaturated hydrocarbons are not found in crude oil to any great
extent. However, they are formed in large amounts in petroleum cracking processes and
have considerable industrial importance.
Naphthene Hydrocarbons
The naphthene hydrocarbons are also called cycloparaffins and, as ther name implies,
they are saturated hydrocarbons in which the carbon chains form closed rings. The
general formula for this series is CnH2n (n greater than 2) and consequently they are
isometric with the olefins. They are named by placing the prefix cyclo before the names
of the corresponding paraffin hydrocarbon. The first members of this series (n=3…6) are
cyclopropane, cyclobutane and cyclohexane, and so on. These compounds, being
saturated, are relatively stable and are important constituents of crude oil. In general, the
chemical properties of these hydrocarbons are very similar to those of the paraffins.
Aromatic Hydrocarbons
These hydrocarbons are also cyclic and may be considered to be derivatives of benzene
and have general formula CnH2n-6 (n greater than 5). Benzene has the formula C6H6, and
the structure consists of a six-fold ring, with alternate single and double bonds. This
structure is so common in organic compounds that chemists use a hexagon with a circle
in the middle as a special symbol to represent the benzene molecule. Some of the simpler
members of this series consist of benzene with one or more alkyl groups as side chains.
An example, methylbenzene, also known as toluene, is of sufficient importance to warrant
a common name.
The fact that the benzene ring contains three double bonds suggests that the members of
this series should be very reactive. However, this is not so and, although they are not as
stable as the paraffins, they do not show the high reactivity that is so characteristic of the olefins. Compounds of this series do occur in crude oil. Petroleum is one of the important
sources of these important hydrocarbons.
The aromatic hydrocarbons are either liquids or solids under standard conditions of
temperature and pressure. Benzene is a colorless liquid with as boiling point of 176oF.
Many of the members of this series are characterized by fragrant odors; hencr the name
aromatic given to this series.